| English |
| adeno-associated virus |
  |
|
| Attestation |
3
|
| Part of speech |
Noun syntagm
|
| Grammatical label |
Countable
|
| Variant |
AAV
|
| Definition |
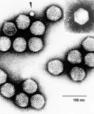
Parvoviridae; icosahedral, 20-25 nm in diameter; single stranded DNA genome with protein capsid. AAV is dependent for replication on presence of wild type adenovirus or herpesvirus; in the absence of helper virus, AAV will stably integrate into the host cell genome. Co-infection with helper virus triggers lytic cycle as do some agents which appropriately perturb host cells. Transduction does not appear to require cell division. Biosafety Level: NIH BSL 2- Pathogenicity: No known pathology for wild type AAV serotype 2. Wild type AAV integrates preferentially into human chromosome 19q13.3-qter; recombinant vectors lose this specificity and appear to integrate randomly, thereby posing a theroretical risk of insertional mutagenesis.
|
| Definition source |
University of California – San Diego. The Program in Human Gene Therapy. Material Safety Data Sheet (MSDS) – Infectious substances. Section 1: Infectious Agent
|
| Context |
Adeno-associated virus (AAV)-mediated expression of a human gamma-globin gene in human progenitor-derived erythroid cells
|
| Context source |
Rund, D. & Rachmilewitz, E. (2000). ‘New Trends in the treatment of beta-thalassemia’. Critical Reviews in Oncology/Hematology 33(2):105-18. (RISCEN189)
|
| Figure source |
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/50130001.htm
|
| Subject field |
Haemopoiesis
|
| Sub-field (level 1) |
Thalassemias
|
| Sub-field (level 2) |
Therapies
|
| Generic concept |
Viral vector
|
| Related concept |
Gene therapy
|
| it |
Adenovirus associato
|
| Reliability code |
3
|